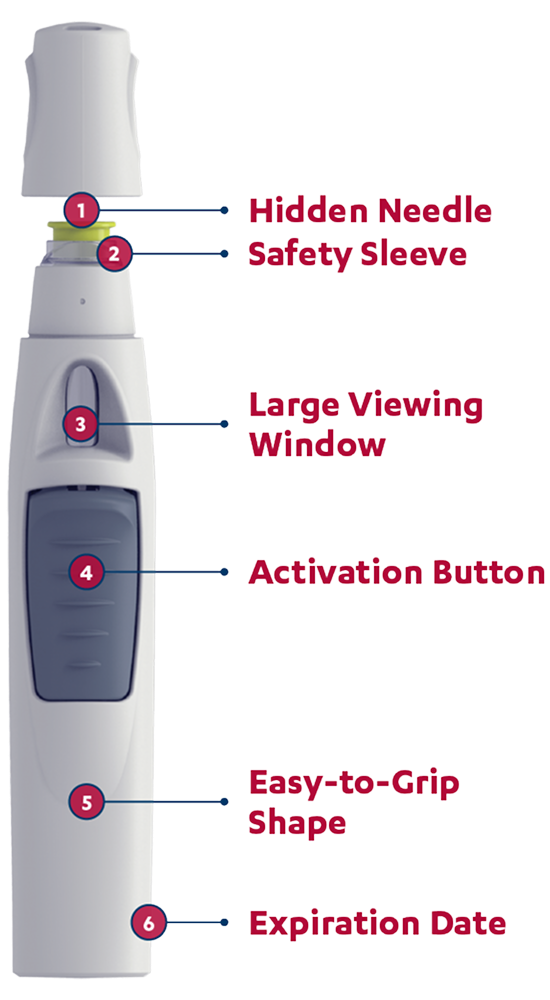
The SmartJect® autoinjector: designed to keep the needle out of sight
Patients will hear a first click when the dose begins to dispense and a second click when the injection is complete and the needle has retracted.
SIMPONI® is intended for use under the guidance and supervision of a physician. Patients may self-inject SIMPONI® after physician approval and proper training in subcutaneous technique.
Prior to initiating SIMPONI®, and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection. Prior to initiating SIMPONI®, patients should be tested for hepatitis B viral infection.

Injection-site reactions
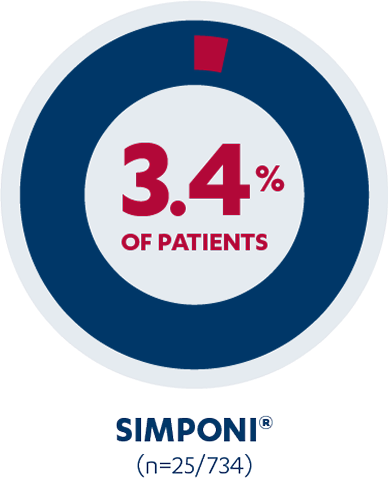
In the PURSUIT induction study, 3.4% of SIMPONI®-treated patients (n=25/734) reported injection-site reactions vs 1.5% of placebo patients (n=5/330). Injection-site reactions included redness, swelling, itching, pain, bruising, and tingling.1,2
In postmarketing experience, serious systemic hypersensitivity reactions (including anaphylactic reaction) have been reported following SIMPONI® administration. Some of these reactions occurred after the first administration of SIMPONI®. If an anaphylactic or other serious allergic reaction occurs, administration of SIMPONI® should be discontinued immediately and appropriate therapy instituted.
Most common adverse reactions are upper respiratory tract infection, nasopharyngitis, and
injection-site reactions.1