MAINTENANCE STUDY
AT 54 WEEKS
PRIMARY ENDPOINT
50% of patients (n=75/151) taking SIMPONI® 100 mg demonstrated sustained clinical response vs 31% of patients (n=48/154) receiving placebo (P<0.001)1,3*†
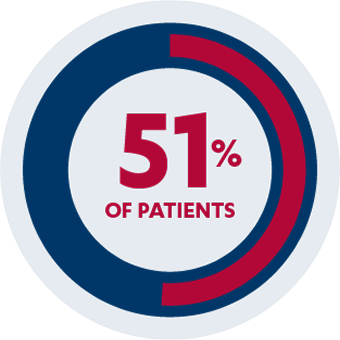
INDUCTION STUDY
AT 6 WEEKS
PRIMARY ENDPOINT
51% of patients (n=129/253) taking SIMPONI® 200 mg/100 mg achieved clinical response vs 30% of patients (n=76/251) receiving placebo (P<0.0001)1,2*

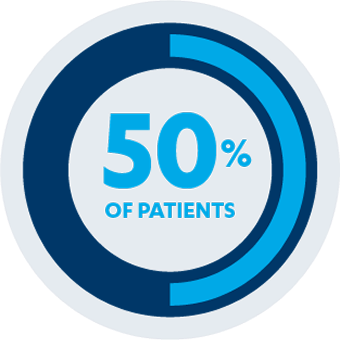
MAINTENANCE STUDY
AT 54 WEEKS
PRIMARY ENDPOINT
50% of patients (n=75/151) taking SIMPONI® 100 mg demonstrated sustained clinical response vs 31% of patients (n=48/154) receiving placebo (P<0.001)1,3*†
The primary endpoint was the percentage of patients in clinical response at Week 6, defined as a decrease from baseline in the Mayo score by ≥30% and ≥3 points, accompanied by a decrease in the rectal bleeding subscore of ≥1 or a rectal bleeding subscore of 0 (no blood seen) or 1 (streaks of blood with stool less than half the time).
The primary endpoint was the percentage of patients maintaining clinical response through Week 54.
The safety and efficacy of SIMPONI® were evaluated in 2 multicenter, randomized, double-blind, placebo-controlled clinical trials in patients ≥18 years of age (Trials UC-1 and UC-2).
Trial UC-1 was a randomized, dose-finding, double-blind, placebo-controlled, multicenter trial conducted in patients with moderately to severely active UC who had an inadequate response or were intolerant to conventional therapy and was divided into 2 parts. In Part 1 (dose finding), patients were randomized to one of 4 treatment groups: 400 mg SIMPONI® administered subcutaneously (SC) at Week 0 and 200 mg at Week 2 (400/200 mg), 200-mg SIMPONI® SC at Week 0 and 100 mg at Week 2 (200/100 mg), 100-mg SIMPONI® SC at Week 0 and 50 mg at Week 2 (100/50 mg), or placebo SC at Weeks 0 and 2. In Part 2 (dose confirming), efficacy was evaluated in 761 patients who were randomized to receive either 400 mg SIMPONI® SC at Week 0 and 200 mg at Week 2, 200-mg SIMPONI® SC at Week 0 and 100 mg at Week 2, or placebo SC at Weeks 0 and 2.
Trial UC-2 was a randomized-withdrawal maintenance trial that evaluated 456 patients who achieved clinical response with SIMPONI® induction and tolerated SIMPONI® treatment. Patients were randomized to receive SIMPONI® 50 mg, SIMPONI® 100 mg or placebo administered subcutaneously every 4 weeks.
Both induction and maintenance studies permitted concomitant treatment with stable doses of oral 5-aminosalicylates (5-ASA), corticosteroids, and/or immunomodulators. However, corticosteroids were to be tapered at the start of the maintenance study in patients in clinical response at Week 6.
For adults with moderately to severely active UC
SIMPONI® requires nearly half the injections compared with Humira® (per first year of treatment)1,4*†
A single 100-mg injection administered subcutaneously every 4 weeks after 3 initial induction injections at Week 0 and Week 21


Explore how the SIMPONI® dosing compares with its competitor.

See What the SIMPONI® Injection Experience Offers Your Patients
The American Gastroenterological Association (AGA) Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis (UC), published in 2024, includes golimumab as a treatment option for adults with moderately to severely active UC. For additional details, click on the link below:
Reference: Singh S, Loftus EV Jr, Limketkai BN, et al. AGA Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2024;167(7):1307-1343. doi:10.1053/
Explore a different treatment option
for adult patients with moderately to severely active ulcerative colitis

Once a decision has been made to prescribe SIMPONI®
J&J withMe Is Your Single Source For Access, Affordability, and Treatment Support for Your Patients
J&J withMe helps verify insurance coverage for your patients, provides reimbursement information, helps find cost support options for eligible patients, and provides ongoing support to help patients start and stay on SIMPONI®. Learn more
Affordability Support for Patients Using Commercial or Private Insurance
The J&J withMe Savings Program can help eligible patients save on their out-of-pocket medicine costs for SIMPONI®.
The patient support and resources provided by J&J withMe are not intended to provide medical advice, replace a treatment plan from the patient’s doctor or nurse, provide case management services, or serve as a reason to prescribe SIMPONI®.