SmartJect® autoinjector: Designed for an easy-to-use experience1
The SmartJect® autoinjector was designed with patients in mind
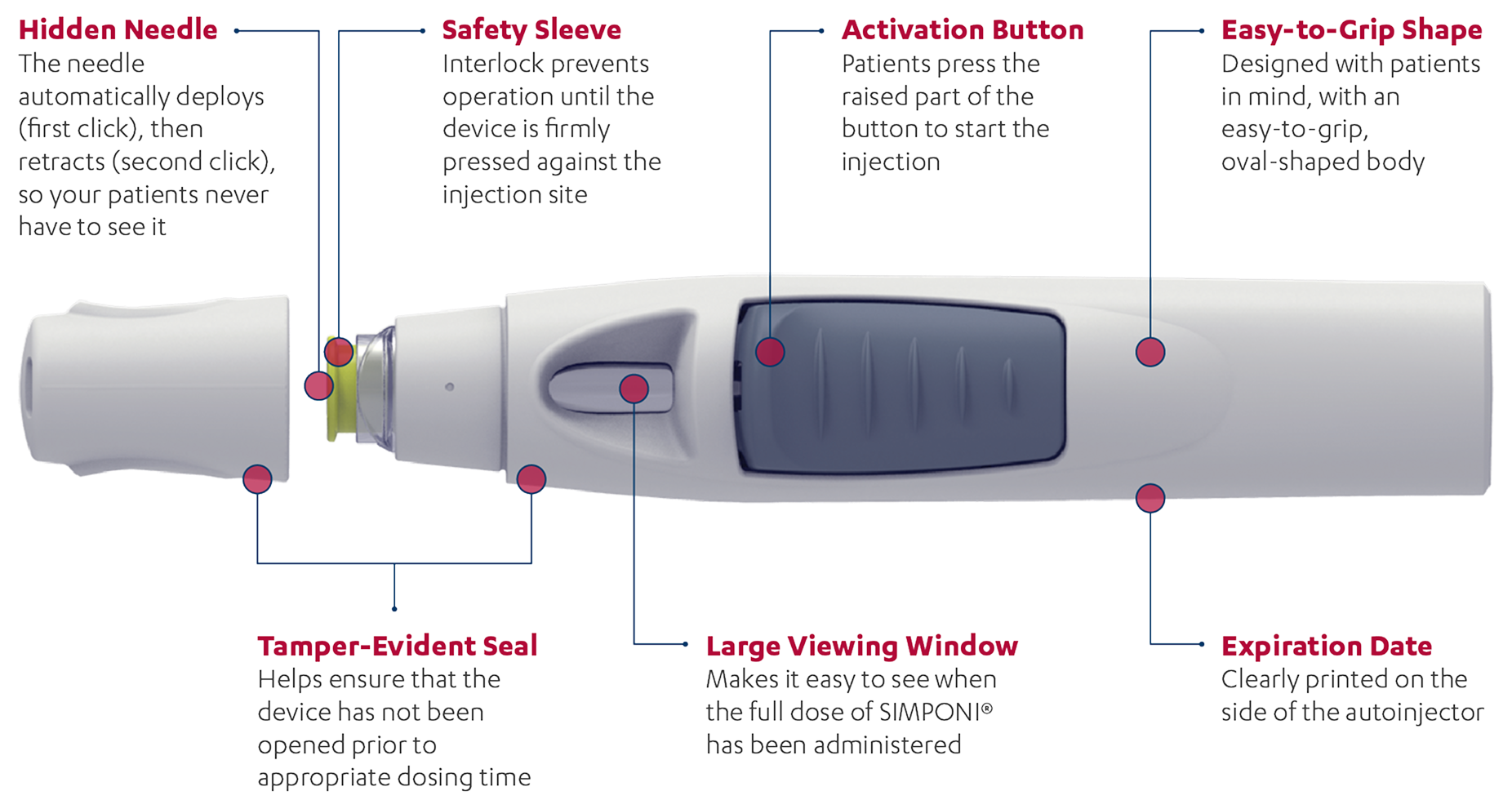

SIMPONI® is intended for use under the guidance and supervision of a physician. Patients may self-inject SIMPONI® after physician approval and proper training.
Prior to initiating SIMPONI®, and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection. Prior to initiating SIMPONI®, patients should be tested for hepatitis B viral infection.
SIMPONI® is also available in a 50 mg/0.5 mL single-dose prefilled syringe.

Injection-site reactions
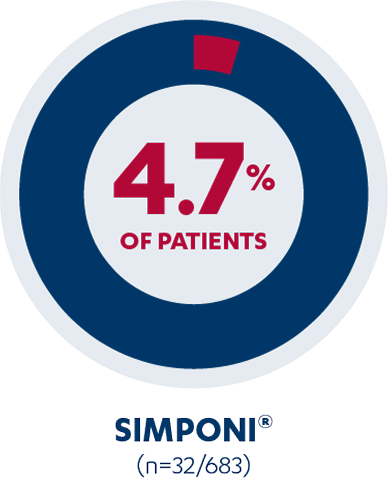
In studies, 4.7% of patients treated with SIMPONI® 50 mg +/- MTX reported injection-site reactions vs 2% of patients treated with placebo +/- MTX. Injection-site reactions included redness, swelling, itching, pain, bruising, and tingling.1,2*†‡
In postmarketing experience, serious systemic hypersensitivity reactions (including anaphylactic reaction) have been reported following SIMPONI® administration. Some of these reactions occurred after the first administration of SIMPONI®. If an anaphylactic or other serious allergic reaction occurs, administration of SIMPONI® should be discontinued immediately and appropriate therapy instituted.
Most common adverse reactions are upper respiratory tract infection, nasopharyngitis, and
injection-site reactions.1
*Data is derived from SIMPONI® 50 mg +/- MTX in adult patients with moderately to severely active RA, active PsA, or active AS at Week 16.2
†Study design: Safety analysis assessed the pooled safety data up to year 3 across five phase 3 trials of SIMPONI® in RA, PsA, and AS.2
‡Patients may have reported ≥1 injection-site reaction.2
References: 1. SIMPONI® (golimumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Kay J, Fleischmann R, Keystone E, et al. Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing
spondylitis. Ann Rheum Dis.
2015;74(3):538-546. doi:10.1136/annrheumdis-2013-20419