once a month1
For patients with RA, SIMPONI® should be given in combination with methotrexate and for patients with PsA or AS, SIMPONI® may be given with or without methotrexate or other nonbiologic Disease-Modifying Antirheumatic Drugs (DMARDs). For patients with RA, PsA, or AS, corticosteroids, non-biologic DMARDs, and/or NSAIDs may be continued during treatment with SIMPONI®.
Per
year
strength
dose SmartJect®
autoinjector1
or
50 mg/0.5 mL single-dose prefilled syringe1
dose SmartJect® autoinjector contains a prefilled glass
syringe providing 50 mg of SIMPONI® per 0.5 mL of
solution. Also available in a
50 mg/0.5 mL single-dose
prefilled syringe.1
under the guidance and supervision of a physician.
Patients may self-inject with SIMPONI® after physician
approval and proper training.
and periodically during
therapy, patients should be
evaluated for active
tuberculosis and tested for
latent infection. Prior to
initiating SIMPONI®, patients should be tested for hepatitis
B viral infection.1
NSAID, nonsteroidal anti-inflammatory drug.
- SIMPONI® autoinjector + MTX1
- Humira® pen3
- Enbrel® autoinjector4
- Minimum number of injections first year
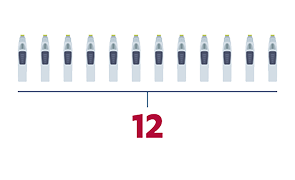
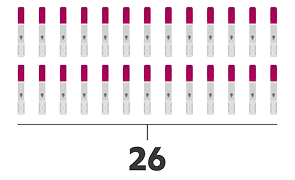
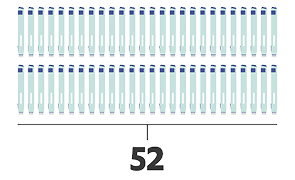
- Dosing schedule for adults with RA, PsA, and AS
- 50 mg once a month
40 mg every other week
Some patients with RA not receiving MTX may benefit from increasing the frequency to 40 mg every week or 80 mg every other week
- 50 mg once a week
- Dosage form and strength
- 50 mg/0.5 mL single-dose prefilled
syringe or single-dose SmartJect®
autoinjector. - 40 mg/0.4 mL and 80 mg/0.8 mL
single-dose Humira® Pen
40 mg/0.4 mL and 80 mg/0.8 mL
single-dose prefilled glass
syringe - 50 mg/mL single-dose prefilled SureClick® autoinjector or single-dose prefilled syringe
- Route of administration
- Subcutaneous injection
- Subcutaneous injection
- Subcutaneous injection
- Indications
- SIMPONI® is indicated for the treatment of adults with moderately to severely active RA, in combination with MTX, adults with active PsA, alone or in combination with MTX, or adults with active AS.
Humira® is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active RA, reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active PsA, and reducing signs and symptoms in adult patients with active AS.
Humira® can be used alone or in combination with MTX or other non-biologic disease-modifying anti-rheumatic drugs (DMARDs) in RA and PsA.
- Enbrel® is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active RA. Enbrel® can be initiated in combination with MTX or used alone. Enbrel® is also indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in adult patients with PsA. Enbrel® can be used with or without MTX. Enbrel® is indicated for reducing signs and symptoms in patients with active AS.
MTX, methotrexate; TNF, tumor necrosis factor.