For adults with moderately to severely active rheumatoid arthritis (RA) in combination with methotrexate
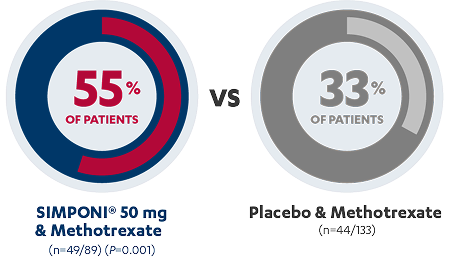
More than half of the patients with RA achieve an ACR 20 response
at Week 14 with SIMPONI® in combination with methotrexate1,2
ACR 20 Response
At Week 14
CO-PRIMARY ENDPOINT1,2

ACR 50 Response
At Week 24
SECONDARY ENDPOINT1,2


CO-PRIMARY ENDPOINT: Improvement from baseline in HAQ-DI score at Week 24: SIMPONI® 50 mg in combination with MTX -0.38 (-0.75 to -0.13) vs MTX alone -0.13 (-0.38 to 0.13) (P<0.001). Overall, 68% of patients receiving SIMPONI® 50 mg in combination with methotrexate achieved a reduction in HAQ-DI score of 0.25 or greater (P<0.001).2
Key Clinical Endpoints from the GO-FORWARD trial evaluating
patients with active RA despite MTX therapy1,2
| Characteristic | Placebo + MTX (N=133) | SIMPONI® 50 mg + MTX (N=89) | P value |
|---|---|---|---|
| Primary endpoints | |||
| ACR 20 at Week 14, n (%) | 44 (33.1%) | 49 (55.1%) | 0.001 |
| HAQ-DI score improvement from baseline at Week 24 | -0.13 (-0.38 to 0.13) | -0.38 (-0.75 to -0.13) | <0.001 |
| Secondary endpoints | |||
| ACR 50 at Week 14, n (%) | 13 (9.8%) | 31 (34.8%) | <0.001 |
| ACR 50 at Week 24, n (%) | 18 (13.5%) | 33 (37.1%) | <0.001 |
ACR 20, American College of Rheumatology 20% improvement; ACR 50, American College of Rheumatology 50% improvement; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate; RA, rheumatoid arthritis.
Improvement in RA Disease Activity1
Patients receiving SIMPONI® with methotrexate demonstrated improvement in individual components of ACR
response criteria compared with methotrexate alone1
GO-FORWARD Trial: Median Percent Improvement From Baseline in the Individual
ACR Components at Week 14*
| Background MTX | SIMPONI® 50 mg + Background MTX | |
|---|---|---|
| N† | 133 | 89 |
| Number of swollen joints (0‑66) | ||
| Baseline | 12 | 13 |
| Week 14 | 38% | 62% |
| Number of tender joints (0‑68) | ||
| Baseline | 21 | 26 |
| Week 14 | 30% | 60% |
| Patient’s assessment of pain (0‑10) | ||
| Baseline | 5.7 | 6.1 |
| Week 14 | 18% | 55% |
| Patient’s global assessment of disease activity (0‑10) | ||
| Baseline | 5.3 | 6.0 |
| Week 14 | 15% | 45% |
| Physician’s global assessment of disease activity (0‑10) | ||
| Baseline | 5.7 | 6.1 |
| Week 14 | 35% | 55% |
| HAQ score (0‑3) | ||
| Baseline | 1.25 | 1.38 |
| Week 14 | 10% | 29% |
| CRP (mg/dL) | ||
| Baseline | 0.8 | 1.0 |
| Week 14 | 2% | 44% |
*In GO-FORWARD trial, about 70% and 85% of patients received concomitant low dose corticosteroids (equivalent to ≤10 mg of prednisone a day) and/or NSAIDs during the trials, respectively.
†N reflects randomized patients; actual number of patients evaluable for each endpoint may vary.
ACR, American College of Rheumatology; CRP, C-reactive protein; HAQ, Health Assessment Questionnaire; NSAID, nonsteroidal anti-inflammatory drug.
SIMPONI® RA Trial Designs1
OBJECTIVE
GO-FORWARD was a multicenter, randomized, double-blind, placebo-controlled study in 444 adult patients who had moderately to severely active rheumatoid arthritis despite a stable dose of ≥15 mg/week of methotrexate (MTX) and who had been previously treated with a TNF (tumor necrosis factor) blocker.
INCLUSION CRITERIA
Moderately to severely active rheumatoid arthritis (RA) was defined as ≥4 swollen joints (out of 66 total) and ≥4 tender joints (out of 68 total) and at least 2 of the following: (1) C-reactive-protein (CRP) ≥1.5 mg/dL or erythrocyte sedimentation rate (ESR) by the Westergren method of ≥28 mm/hr, (2) ≥30 minutes of morning stiffness, (3) bone erosion determined by X-ray and/or MRI (magnetic resonance imaging), or (4) anti-cyclic citrullinated peptide (anti-CCP) antibody-positive or rheumatoid factor (RF)-positive.
TREATMENT REGIMEN
Patients were randomized to receive placebo + MTX (n=133), SIMPONI® 50 mg + MTX (n=89), SIMPONI® 100 mg + MTX (n=89), or SIMPONI® 100-mg monotherapy + placebo + MTX (n=133). At Week 16, patients with <20% improvement from baseline in both tender and swollen joint counts had their study medication adjusted in a double-blind fashion (ie, early escape). Early-escape patients in the placebo + MTX group began receiving SIMPONI® 50 mg every 4 weeks. Early-escape patients in the SIMPONI® 50 mg + MTX group had their SIMPONI® dose increased from 50 mg every 4 weeks to 100 mg every 4 weeks. Both groups continued to receive MTX. At Week 24, all patients remaining in the placebo + MTX group who had been receiving placebo injections began receiving SIMPONI® 50 mg in a blinded fashion. Because the 100-mg dose is not part of the approved dosing regimen for SIMPONI®, only 50-mg results are shown. There was no clear evidence of improved ACR response with the higher SIMPONI® dose group (100 mg).
EFFICACY ENDPOINTS
The primary endpoint was the percentage of patients achieving an ACR 20 (American College of Rheumatology 20%) response at Week 14.
References: 1. SIMPONI® (golimumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc. 2. Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68(6):789-796. doi:10.1136/ard.2008.099010
3. Data on file. Janssen Biotech, Inc.
For adults with moderately to severely active rheumatoid arthritis (RA) in combination with methotrexate
Demonstrated Safety Profile for SIMPONI® in RA3
Select adverse events (AEs) through Week 52 in RA Phase 3 clinical trials3*
| Adverse Reaction | SIMPONI® (combined) ± DMARDs† | Placebo ± DMARDs |
|---|---|---|
| Patients in Phase 3 RA trials, n | 1463 | 449 |
| Average follow-up, weeks | 66.9 | 30.2 |
| Patients with ≥1 AE, % (n) | 87.2 (1276) | 75.9 (341) |
| Patients with ≥1 serious AE, % (n) | 16.1 (236) | 9.6 (43) |
| Infections and infestations, % (n) | 60.4 (883) | 37.0 (166) |
| Serious infections and infestations, % (n) | 5.3 (77) | 2.7 (12) |
| Most common AEs (occurring in ≥5% of patients with SIMPONI®) | ||
| Infections and infestations, % (n) | 60.4 (883) | 37.0 (166) |
| Upper respiratory tract infection, % (n) | 18.2 (266) | 9.1 (41) |
| Nasopharyng- itis, % (n) | 11.0 (161) | 6.0 (27) |
| Bronchitis, % (n) | 8.6 (126) | 4.9 (22) |
| Sinusitis, % (n) | 7.0 (103) | 3.1 (14) |
| Urinary tract infection, % (n) | 5.5 (80) | 3.8 (17) |
| Pharyngitis, % (n) | 5.0 (73) | 3.1 (14) |
| Gastro- intestinal disorders, % (n) | 36.3 (531) | 27.6 (124) |
| Nausea, % (n) | 11.2 (164) | 9.4 (42) |
| Diarrhea, % (n) | 7.5 (110) | 6.2 (28) |
| Musculo- skeletal and connective tissue disorders, % (n) | 30.1 (144) | 20.9 (94) |
| RA, % (n) | 7.2 (106) | 5.3 (24) |
| Back pain, % (n) | 6.0 (88) | 2.9 (13) |
| Arthralgia, % (n) | 5.5 (80) | 3.6 (16) |
| General disorders and administration-site conditions, % (n) | 26.4 (386) | 15.6 (70) |
| Injection-site erythema, % (n) | 7.1 (104) | 1.1 (5) |
| Respiratory, thoracic, mediastinal disorders, % (n) | 22.7 (332) | 15.6 (70) |
| Cough, % (n) | 9.9 (145) | 5.8 (26) |
| Skin and subcutaneous tissue disorders, % (n) | 21.2 (310) | 15.6 (70) |
| Rash, % (n) | 5.6 (82) | 4.2 (19) |
| Nervous system disorders, % (n) | 19.2 (281) | 11.1 (50) |
| Headache, % (n) | 7.8 (114) | 6.0 (27) |
| Investigat- ions, % (n) | 18.3 (267) | 12.0 (54) |
| ALT increased, % (n) | 8.1 (118) | 5.8 (26) |
| AST increased, % (n) | 5.5 (81) | 3.8 (17) |
| Injury, poisoning, and procedural complications, % (n) | 16.1 (236) | 8.7 (39) |
| Vascular disorders, % (n) | 11.3 (165) | 4.9 (22) |
| Hypertension, % (n) | 7.3 (107) | 2.4 (11) |
| Psychiatric disorders, % (n) | 8.7 (128) | 6.0 (27) |
| Metabolism and nutrition disorders, % (n) | 7.7 (112) | 4.9 (22) |
| Blood and lymphatic system disorders, % (n) | 6.2 (91) | 4.5 (20) |
*As of June 2008, 75% patients had golimumab exposure ≥52 weeks.
†Safety in all patients treated with golimumab from the first dose of golimumab through the last safety visit. Patients may appear in more than 1 column.
References: 1. SIMPONI® (golimumab) [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
2. Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68(6):789-796. doi:10.1136/ard.2008.099010
3. Data on file. Janssen Biotech, Inc.