For adults with moderately to severely active rheumatoid arthritis (RA) in combination with methotrexate
More than half of the patients with RA achieve an ACR 20 response at Week 14 with SIMPONI® in combination with methotrexate1,2
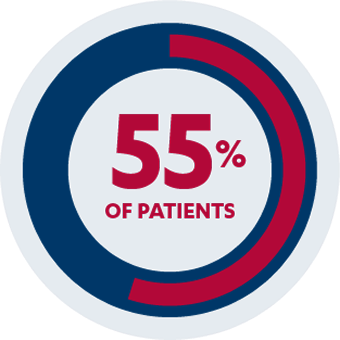
AT WEEK 14
Co-Primary Endpoint1,2
55% of patients (n=49/89) receiving SIMPONI® 50 mg with methotrexate achieved an ACR 20 response vs 33% of patients (n=44/133) receiving methotrexate alone (P=0.001)
AT WEEK 24
Co-Primary Endpoint1,2
Improvement from baseline in HAQ-DI score at Week 24: SIMPONI® 50 mg in combination with MTX -0.38 (-0.75 to -0.13) vs MTX alone -0.13 (-0.38 to 0.13) (P<0.001)
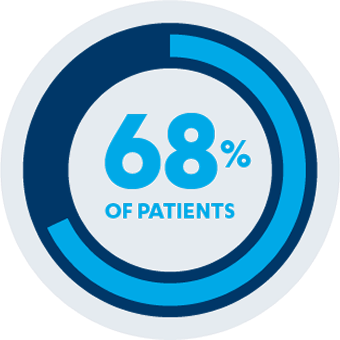
68% of patients receiving SIMPONI® in combination with methotrexate had reduced HAQ-DI scores from baseline at Week 24 (P<0.001)2*
The co-primary endpoints were the percentage of patients achieving an ACR 20 response at Week 14 and the improvement from baseline in HAQ-DI score at Week 24. One of the secondary endpoints was the percentage of patients achieving an ACR 50 response at Week 24.2
More patients with RA achieve an ACR 50 response at Week 14 with SIMPONI® in combination with methotrexate1,2
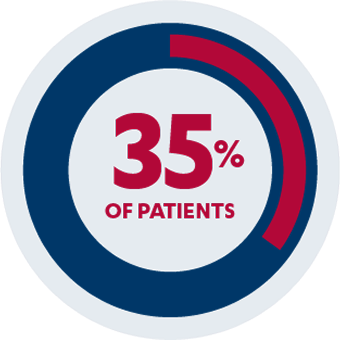
AT WEEK 14
Secondary Endpoint1,2
35% of patients (n=31/89) receiving SIMPONI® 50 mg with methotrexate achieved an ACR 50 response vs 10% of patients (n=13/133) receiving methotrexate alone (P<0.001)
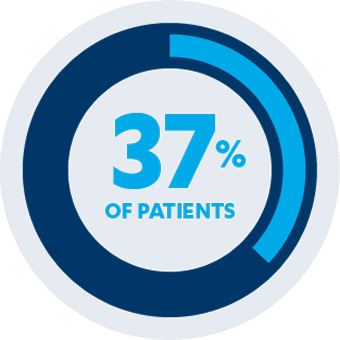
AT WEEK 24
Secondary Endpoint1,2
37% of patients (n=33/89) receiving SIMPONI® 50 mg with methotrexate sustained an ACR 50 response vs 14% of patients (n=18/133) receiving methotrexate alone (P<0.001)
STUDY DESIGN
GO-FORWARD was a multicenter, randomized, double-blind, placebo-controlled study in 444 adult patients who had moderately to severely active rheumatoid arthritis despite a stable dose of ≥15 mg/week of methotrexate (MTX) and who had been previously treated with a TNF (tumor necrosis factor) blocker. Moderately to severely active rheumatoid arthritis (RA) was defined as ≥4 swollen joints (out of 66 total) and ≥4 tender joints (out of 68 total) and at least 2 of the following: (1) C-reactive-protein (CRP) ≥1.5 mg/dL or erythrocyte sedimentation rate (ESR) by the Westergren method of ≥28 mm/hr, (2) ≥30 minutes of morning stiffness, (3) bone erosion determined by X-ray and/or MRI (magnetic resonance imaging), or (4) anti-cyclic citrullinated peptide (anti-CCP) antibody-positive or rheumatoid factor (RF)-positive. Patients were randomized to receive placebo + MTX (n=133), SIMPONI® 50 mg + MTX (n=89), SIMPONI® 100 mg + MTX (n=89), or SIMPONI® 100-mg monotherapy + placebo + MTX (n=133). At Week 16, patients with <20% improvement from baseline in both tender and swollen joint counts had their study medication adjusted in a double-blind fashion (ie, early escape). Early-escape patients in the placebo + MTX group began receiving SIMPONI® 50 mg every 4 weeks. Early-escape patients in the SIMPONI® 50 mg + MTX group had their SIMPONI® dose increased from 50 mg every 4 weeks to 100 mg every 4 weeks. Both groups continued to receive MTX. At Week 24, all patients remaining in the placebo + MTX group who had been receiving placebo injections began receiving SIMPONI® 50 mg in a blinded fashion. Because the 100-mg dose is not part of the approved dosing regimen for SIMPONI®, only 50-mg results are shown. There was no clear evidence of improved ACR response with the higher SIMPONI® dose group (100 mg).1,2
For adults with moderately to severely active RA in combination with MTX, active PsA alone or in combination with MTX, or active AS
SIMPONI® requires fewer than half the number of injections compared with Humira®1,3*†
A single 50-mg dose administered subcutaneously once a month1

Explore how SIMPONI® dosing compares with its competing products.

See What the SIMPONI® Injection Experience Offers Your Patients

Once a decision has been made to prescribe SIMPONI®
J&J withMe Is Your Single Source For Access, Affordability, and Treatment Support for Your Patients
J&J withMe helps verify insurance coverage for your patients, provides reimbursement information, helps find cost support options for eligible patients, and provides ongoing support to help patients start and stay on SIMPONI®. Learn more
Affordability Support for Patients Using Commercial or Private Insurance
The J&J withMe Savings Program can help eligible patients save on their out-of-pocket medicine costs for SIMPONI®.
The patient support and resources provided by J&J withMe are not intended to provide medical advice, replace a treatment plan from the patient’s doctor or nurse, provide case management services, or serve as a reason to prescribe SIMPONI®.

