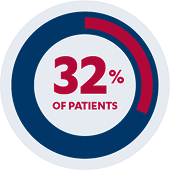
Primary Endpoint
32% of pediatric patients (n=21/66) receiving SIMPONI® were in clinical remission*†
The primary endpoint was clinical remission at Week 6 as measured by the Mayo score (defined as ≤2 points, with no individual subscore > 1).
Secondary Endpoint

Additional Secondary Endpoints

patients (n=12/21)*†§
*See complete study design for endpoint definitions and trial details.
†Clinical remission is defined as a Mayo score of ≤2 points, with no individual subscore >1.
‡Clinical response is defined as a decrease from baseline in the Mayo score by ≥30% and ≥3 points, with either a decrease from baseline in the rectal bleeding subscore of ≥1 or a rectal bleeding subscore of 0 or 1.
§Endpoint is evaluated among patients in clinical remission at Week 6.
The efficacy and safety of SIMPONI® was evaluated in a multi-center, open-label pediatric study of 69 patients. Efficacy was assessed in 66 patients weighing at least 15 kg with moderately to severely active ulcerative colitis (UC).
Patients presented with a Mayo score of 6 to 12 with an endoscopy subscore of ≥2 who had an inadequate response to corticosteroids, 6-mercaptopurine
(6-MP) or azathioprine (AZA), or who were intolerant to or had medical contraindications for such therapies. Patients with prior exposure to tumor necrosis factor (TNF) blockers were ineligible for participation.
Patients weighing 15 kg to less than 45 kg received SIMPONI® subcutaneously 120 mg/m2 at Week 0, 60 mg/m2 at Week 2, and 60 mg/m2 every 4 weeks from Week 6 onward. Patients weighing at least 45 kg received SIMPONI® 200 mg at Week 0, 100 mg at Week 2, and 100 mg every 4 weeks from Week 6 onward. The recommended body-weight tiered dosage for pediatric patients weighing 15 kg to less than 40 kg and 40 kg to less than 45 kg differs from the body surface area-based dosage administered in this study.
There are no anticipated clinically relevant differences in efficacy between the recommended and studied pediatric dosages of SIMPONI®.
The primary endpoint was clinical remission at Week 6 as measured by the Mayo score (defined as ≤2 points, with no individual subscore >1). Secondary endpoint was clinical response at Week 6 (defined as a decrease from baseline in the Mayo score by ≥30% and ≥3 points, with either a decrease from baseline in the rectal bleeding subscore of ≥1 or a rectal bleeding subscore of 0 or 1). Additional secondary endpoints included clinical remission and maintenance of clinical remission at Week 54.
The Safety of SIMPONI® Has Been Established in the PURSUIT-2 Open-Label Phase 3 Study
Treatment-Emergent Adverse Events of SIMPONI® in the PURSUIT-2 Open-Label Phase 3 Study
Through Week 6 and Week 541
| Adverse Events | Induction Phase Weeks 0-6 (N=69) | Mainten- ance Phase Weeks |
|---|---|---|
| Average duration of follow-up (weeks) | 6.3 | 40.0 |
| Average exposure (number of administ- rations) | 2.0 | 9.0 |
| Patients with ≥1, n (%)†‡ | ||
| AEs | 47 (68.1) | 58 (93.5) |
| Serious AEs | 10 (14.5) | 21 (33.9) |
| AEs leading to death | 0 | 0 |
| AEs leading to discontinu- ation | 6 (8.7) | 9 (14.5) |
| Infections | 17 (24.6) | 38 (61.3) |
| Serious infections | 1 (1.4)§ | 9 (14.5)|| |
| Malignant neoplasms | 0 | 0 |
| Injection-site reactions | 2 (2.9) | 3 (4.8) |
*Included patients who received ≥1 dose (complete or partial) of SIMPONI® during the maintenance phase.1
†AEs were coded using MedDRA version 26.1.1
‡Patients were only counted once for any given event, regardless of the number of times they experienced the event.1
§One case of pseudomembranous colitis.1
||Two cases each of cytomegalovirus colitis and pneumonia, and one case each of Clostridium difficile infection, COVID-19, stump appendicitis, and fungal test positive (candida). One case of “UC worsening” was classified as “infection” though the investigator later confirmed no evidence of infection was found.1