Discover a treatment to help pediatric patients reach and maintain remission with every 4 week subcutaneous administration after 2 or 3 induction injections depending on body weight.

Discover a treatment to help pediatric patients reach and maintain remission with every 4 week subcutaneous administration after 2 or 3 induction injections depending on body weight.


for more information
SIMPONI® Clinical Remission at Week 6 in PURSUIT-2 Open-Label Phase 3 Study
AT WEEK 6
Primary Endpoint
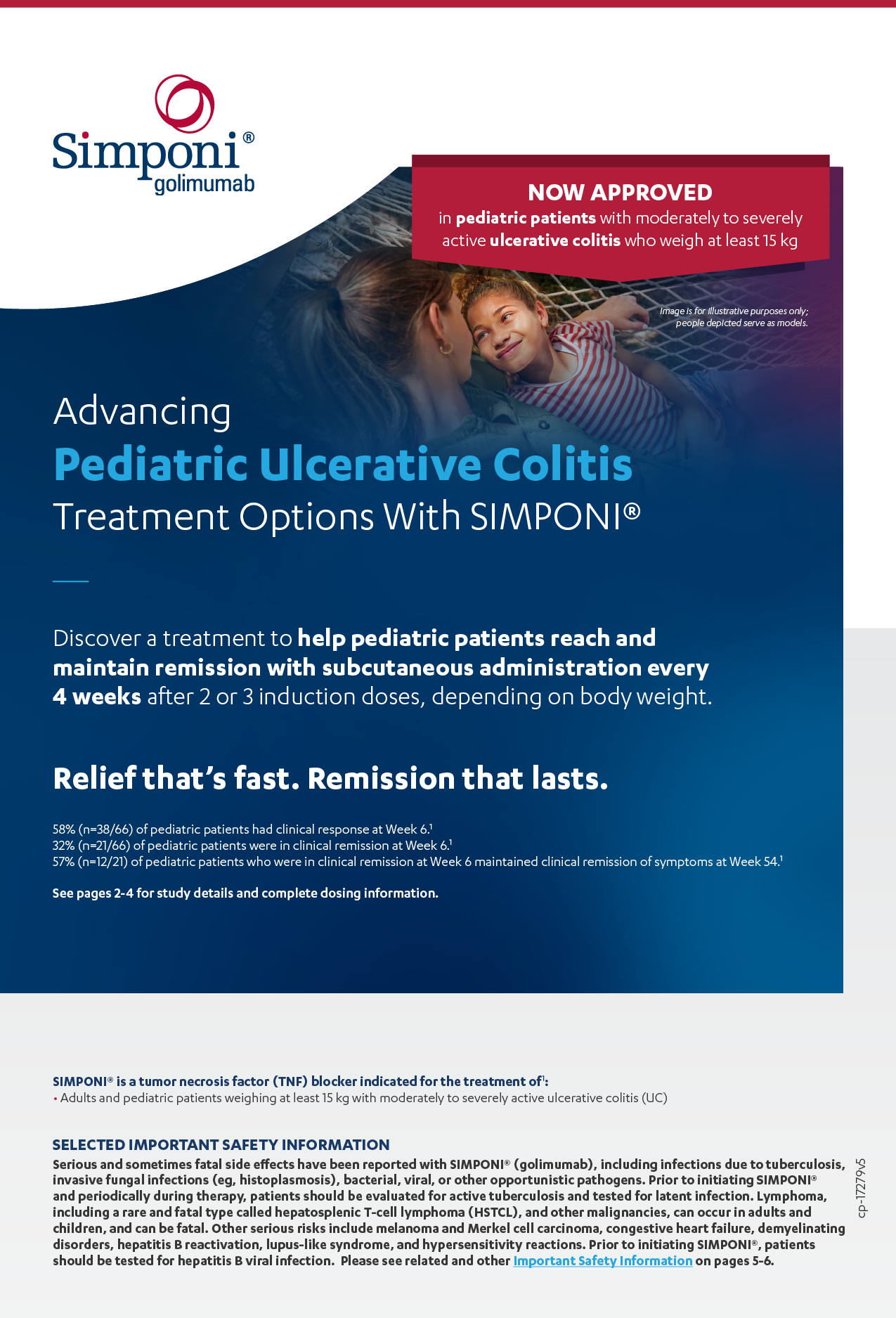
32% of pediatric patients (n=21/66) receiving SIMPONI® were in clinical remission*†
Primary Endpoint
The primary endpoint was clinical remission at Week 6 as measured by the Mayo score (defined as ≤2 points, with no individual subscore >1).
More Than Half of Pediatric Patients Receiving SIMPONI® Were Observed to Have Fast Response and Sustained Clinical Remission1
In the PURSUIT 2 open-label phase 3 study that evaluated patients weighing at least 15 kg with moderately to severely active UC:
AT 6 WEEKS
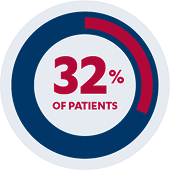
Secondary Endpoint
Clinical response: 58% of pediatric patients (n=38/66)*‡
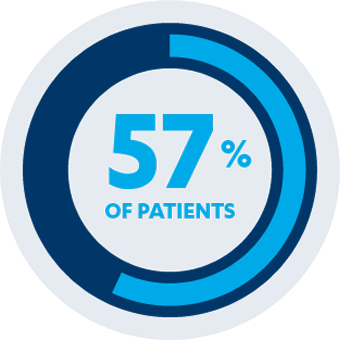
AT 54 WEEKS
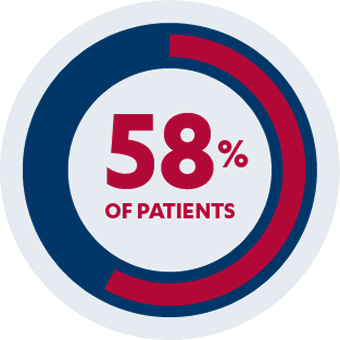
ADDITIONAL SECONDARY ENDPOINTS
Maintenance of clinical remission: 57% of pediatric patients (n=12/21)*†§
Clinical remission: 34% of pediatric patients (n=13/38) were in clinical remission among those who had clinical response at Week 6.*†
STUDY DESIGN
The efficacy and safety of SIMPONI® was evaluated in a multi-center, open-label pediatric study of 69 patients. Efficacy was assessed in 66 patients weighing at least 15 kg with moderately to severely active ulcerative colitis (UC). Patients presented with a Mayo score of 6 to 12 with an endoscopy subscore of ≥2 who had an inadequate response to corticosteroids, 6-mercaptopurine (6-MP) or azathioprine (AZA), or who were intolerant to or had medical contraindications for such therapies. Patients with prior exposure to tumor necrosis factor (TNF) blockers were ineligible for participation. Patients weighing 15 kg to less than 45 kg received SIMPONI® subcutaneously 120 mg/m2 at Week 0, 60 mg/m2 at Week 2, and 60 mg/m2 every 4 weeks from Week 6 onward. Patients weighing ≥45 kg received SIMPONI® 200 mg at Week 0, 100 mg at Week 2, and 100 mg every 4 weeks from Week 6 onward. The recommended body-weight tiered dosage for pediatric patients weighing 15 to less than 40 kg and 40 to less than 45 kg differs from the body surface area-based dosage administered in this study. There are no anticipated clinically relevant differences in efficacy between the recommended and studied pediatric dosages of SIMPONI®.
For pediatric patients weighing at least 15 kg with moderately to severely active ulcerative colitis (UC)
SIMPONI® requires nearly half the injections compared with Humira® (per first year of treatment)1,2*†
(88 lbs) or greater, the recommended Day 1 dosage is 160 mg, the Day 8 dosage is 80 mg, the Day 15 dosage is 80 mg, and beginning on Day 29 the dosage is 40 mg every week or 80 mg every other week. The total number of injections (including both induction and maintenance) during the first year of treatment for pediatric patients receiving 1 maintenance injection every 2 weeks is 28 or 29 injections. The total number of injections (including both induction and maintenance) during the first year of treatment for pediatric patients receiving 1 maintenance injection every week is 52 or 53 injections.2
Pediatric dosage for SIMPONI® is based on body weight requiring a single injection administered subcutaneously every 4 weeks after 2 or 3 induction injections

Explore how the SIMPONI® dosing compares with its competitor.
See What the SIMPONI® Injection Experience Offers Your Pediatric Patients

Once a decision has been made to prescribe SIMPONI®
J&J withMe Is Your Single Source For Access, Affordability, and Treatment Support for Your Patients
J&J withMe helps verify insurance coverage for your patients, provides reimbursement information, helps find cost support options for eligible patients, and provides ongoing support to help patients start and stay on SIMPONI®. Learn more
Affordability Support for Patients Using Commercial or Private Insurance
The J&J withMe Savings Program can help eligible patients save on their out-of-pocket medicine costs for SIMPONI®.
The patient support and resources provided by J&J withMe are not intended to provide medical advice, replace a treatment plan from the patient’s doctor or nurse, provide case management services, or serve as a reason to prescribe SIMPONI®.

